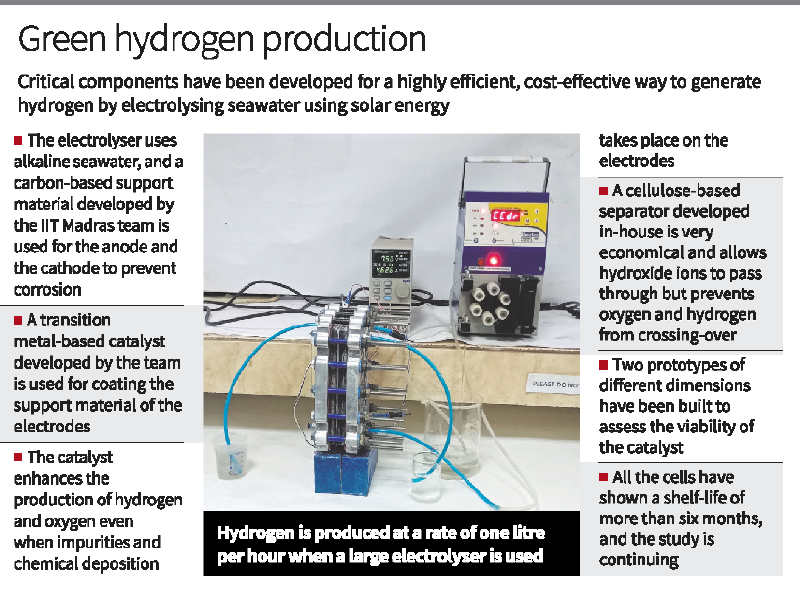
Researchers from the Department of Physics at IIT-Madras have made significant advancements in the generation of green hydrogen by developing a cost-effective and efficient method to electrolyze seawater using solar energy. The team, led by Dr. Ramaprabhu Sundara, addressed several challenges associated with traditional alkaline water electrolysis, such as high energy consumption, expensive materials, and freshwater usage.
The researchers replaced freshwater with alkaline seawater and utilized carbon-based support material for the electrodes, minimizing the risk of corrosion. They also designed transition metal-based catalysts capable of catalyzing both oxygen and hydrogen evolution reactions, even in the presence of impurities. Additionally, they developed a cellulose-based separator that allows the passage of hydroxide ions while preventing crossover of oxygen and hydrogen.
By optimizing various parameters, the team achieved a voltage of 1.73 V at a current density of 10 mA/sq.cm for seawater electrolysis, using photovoltaic-derived voltage. The researchers built prototypes of different dimensions, demonstrating hydrogen production rates of 250 ml per hour and 1 liter per hour in smaller and larger electrolyzers, respectively. They also assembled a stack of three cells, producing approximately four liters of hydrogen per hour.
This breakthrough research paves the way for highly efficient and cost-effective electrolysis of seawater to generate green hydrogen. The use of solar energy and the innovative design of carbon-based electrodes, transition metal catalysts, and cellulose-based separators contribute to sustainable hydrogen production from abundant seawater resources.
FOR MORE DETAILS REFERS HINDU